
DMT-Nexus member
Posts: 316 Joined: 02-Oct-2009 Last visit: 10-Nov-2012 Location: The White Visitation
|
After SWIM's first experience with xylene, he swore off solvents & decided he'd stick with good old d-Limonene and IPA when necessary. Now, however, he's realizing he might need to use solvents in the future (particularly MEK for purifying mescaline and naptha for obtaining high-purity DMT). Since he has to use them, he wants them to be as safe as possible. As such, he's interested in distilling them. But while he's familiar with the basics of distillation, he's not too clear on exactly what kind apparatus he'd need to make his own pure MEK & naptha. Could he use a distillation apparatus like this one: 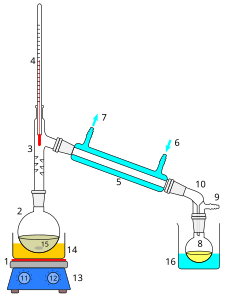 or is this too complex? Would it be possible with something simpler? Also, would this kind of setup create much of a smell in his condo if he used it to distill MEK/naptha? benzyme wrote:
i'm tellin ya, one day i'll interface a mass spec and uv-vis spectrophotometer to a modular synthesizer
|
|
|
|
|

DMT-Nexus member
Posts: 557 Joined: 09-Sep-2009 Last visit: 26-Jun-2012
|
Thats exactly what swiy would need however make sure the system has an opening so that pressure doesnt build up and the system does not become a bomb. Swiy needs a heat source (ie mantle, bowl of sand or oil on hotplate), water source, flask, stillhead, condensor, and a reciever and recieving flask. The reciever can be ground glass but with a vacuum opening or non ground to avoid and pressure buildup. Swim would suggest you look at the mit chemistry videos (in a sticky somewhere in the nexus) to give you a better idea of how a distillation is set up and performed safely.
|
|
|

DMT-Nexus member
Posts: 316 Joined: 02-Oct-2009 Last visit: 10-Nov-2012 Location: The White Visitation
|
swim's main concern was with solvent vapors leaking out through the openings (that necessary to avoid pressure buildup) & stinking up his place, but a filter flask sounds like it might not let that much out. thx for the reply benzyme wrote:
i'm tellin ya, one day i'll interface a mass spec and uv-vis spectrophotometer to a modular synthesizer
|
|
|

DMT-Nexus member
Posts: 557 Joined: 09-Sep-2009 Last visit: 26-Jun-2012
|
If swiy is using ground glass lab gear there will be no leaks, and if the cooling water in the condensor is doing its job swiy shouldnt experiance any solvent gases. Sure they will still smell a bit but not as much as they would otherwise.
|
|
|

DMT-Nexus member
Posts: 57 Joined: 17-Nov-2013 Last visit: 09-Oct-2022 Location: Cali
|
I give you life...lol.
Swims is considering doing the same thing with naphtha but was wondering if a vigrux column would be necessary. Swims has no concern with isolating different fractions in the naphtha. He is just attempting to make a solvent that will undoubtedly fully evaporate from the final product.
|
|
|

analytical chemist
   
Posts: 7463 Joined: 21-May-2008 Last visit: 09-Aug-2025 Location: the lab
|
then you don't need a vigreux column, just a liebig condenser "Nothing is true, everything is permitted." ~ hassan i sabbah
"Experiments are the only means of attaining knowledge at our disposal. The rest is poetry, imagination." -Max Planck
|
|
|

Boundary condition

Posts: 8617 Joined: 30-Aug-2008 Last visit: 24-Dec-2025 Location: square root of minus one
|
Liebig condensers are great, although I once distilled a heavy hydrotreated naphtha using 5 feet of straight 15 mm copper pipe as a condenser. I did use a distillation flask with a sidearm, though. The sidearm was sealed into the pipe using a rubber grommet. It was a bit ghetto-meets-labware and I wouldn't do it with low-boiling-point solvents. Sometimes I reflect on how fortunate I am to still be alive... If you can solder pipes, making a copper Liebig condenser is fairly easy. “There is a way of manipulating matter and energy so as to produce what modern scientists call 'a field of force'. The field acts on the observer and puts him in a privileged position vis-à-vis the universe. From this position he has access to the realities which are ordinarily hidden from us by time and space, matter and energy. This is what we call the Great Work." ― Jacques Bergier, quoting Fulcanelli
|