
DMT-Nexus member
Posts: 121 Joined: 05-May-2008 Last visit: 24-Jan-2025 Location: 2nd star to the right
|
Would a 'manske' style salting method work on crude mimosa tea ? The idea here not to end up with pure dmt but just a crude extract.
So extracting using cold water + vinegar. Then adding salt to the extracted fluid. Then filtering out whatever precipitates.
The interest in this is - not to have to drink a mimosa tea containing vinegar, but instead ending up with a crude resin that still contains the full alkaloid profile of mimosa.
Would this work ?
|
|
|
|
|

Rennasauce Man
Posts: 853 Joined: 27-May-2011 Last visit: 28-Feb-2025 Location: A Pale Blue Dot orbiting a GV2 Yellow Dwarf fusion powered Luminous Ball of Plasma at 30km/s
|
Hey idk, but if you try it first, ill give it a go as well with fumaric acid. That would be really cool if it did work. Just found this from back in the day- https://www.dmt-nexus.me....aspx?g=posts&t=3859"let those who have talked to the elves, find each other and band together" -TMK
In a society in which nearly everybody is dominated by somebody else's mind or by a disembodied mind, it becomes increasingly difficult to learn the truth about the activities of governments and corporations, about the quality or value of products, or about the health of one's own place and economy.
In such a society, also, our private economies will depend less upon the private ownership of real, usable property, and more upon property that is institutional and abstract, beyond individual control, such as money, insurance policies, certificates of deposit, stocks, etc. And as our private economies become more abstract, the mutual, free helps and pleasures of family and community life will be supplanted by a kind of displaced citizenship and by commerce with impersonal and self-interested suppliers...
The great enemy of freedom is the alignment of political power with wealth. This alignment destroys the commonwealth - that is, the natural wealth of localities and the local economies of household, neighborhood, and community - and so destroys democracy, of which the commonwealth is the foundation and practical means.” - Wendell Berry
|
|
|

DMT-Nexus member
 
Posts: 12340 Joined: 12-Nov-2008 Last visit: 02-Apr-2023 Location: pacific
|
I am unsure of converting DMT acetate to HCL without basing it after the acetate pull..beyond that acetates I know for sure, and most other salts of DMT are water soluble..so how would you expect them to drop out? Maybe super cold temps might do it I cant really say. Long live the unwoke.
|
|
|

Rennasauce Man
Posts: 853 Joined: 27-May-2011 Last visit: 28-Feb-2025 Location: A Pale Blue Dot orbiting a GV2 Yellow Dwarf fusion powered Luminous Ball of Plasma at 30km/s
|
Maybe a good place to start would be to try a mini-manske on some already extracted dmt acetate, fumatate, etc. If you know it does not work with a clean solution, then it for sure will not work with a filtered brew. "let those who have talked to the elves, find each other and band together" -TMK
In a society in which nearly everybody is dominated by somebody else's mind or by a disembodied mind, it becomes increasingly difficult to learn the truth about the activities of governments and corporations, about the quality or value of products, or about the health of one's own place and economy.
In such a society, also, our private economies will depend less upon the private ownership of real, usable property, and more upon property that is institutional and abstract, beyond individual control, such as money, insurance policies, certificates of deposit, stocks, etc. And as our private economies become more abstract, the mutual, free helps and pleasures of family and community life will be supplanted by a kind of displaced citizenship and by commerce with impersonal and self-interested suppliers...
The great enemy of freedom is the alignment of political power with wealth. This alignment destroys the commonwealth - that is, the natural wealth of localities and the local economies of household, neighborhood, and community - and so destroys democracy, of which the commonwealth is the foundation and practical means.” - Wendell Berry
|
|
|
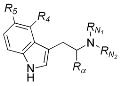
DMT-Nexus member
Posts: 253 Joined: 06-Jul-2010 Last visit: 11-Sep-2011 Location: Never Neverland
|
I really don't think it would work, I think the manske is a harmala-alkaloid-only process.
|
|
|

DMT-Nexus member
Posts: 121 Joined: 05-May-2008 Last visit: 24-Jan-2025 Location: 2nd star to the right
|
I think this post is just going to illustrate my lack of chem know how.....
rather than aiming to create dmt hcl, was hoping that the water would just become so saturated with sodium chloride that it would sort push out the dmt acetate......
but that would only happen if dmt acetate is less soluble than sodium chloride right?.....looking at a solubility table it seems that acetates in general are pretty water soluble......so not an effective method of precipitation........
while on this topic though (maybe not the proper place for it but...) ..... i can't find mentioned in the forum of anyone performing a straight vinegar & sodium carbonate extraction on mimosa......so soaking some powdered MHRB in pure 5% distilled vinegar and then adding carbonate, filtering and collecting the mud.......would all the spice end up in the mud or would some still remain in the water ? (the idea here, again, being not to create the purest product but just something a bit more concentrated and less nauseating than a tea)
SWIM has tried drinking the water (because he thought all the dmt would remain in the water and only the tannins and other crud would fall to the bottom) of such a reaction with mixed results.....if drunk a day or so after adding sodium bicarb (so not sodium carbonate)....it is still active, but such a mixture left in the fridge for several weeks makes the water inactive...........any thoughts? (again sorry if this is the wrong place....saw 'salting' and thought it was relevant)
|
|
|

DMT-Nexus member
Posts: 190 Joined: 24-Jan-2011 Last visit: 21-Mar-2013 Location: My body for now
|
I have had MHRB tea in the fridge for 6 months now, I have not noticed a potency drop, just the tea getting clearer and clearer. About once a month I will change out the jar it is stored in because plant material is still dropping out of it - very thin layers attaching to the glass, bottom and up the sides partially. This tea was boiled with citric and ascorbic acids and filter several times. The truth is not for all men, but only for those who seek it.
|
|
|

DMT-Nexus member
Posts: 121 Joined: 05-May-2008 Last visit: 24-Jan-2025 Location: 2nd star to the right
|
stick it in the freezer for a day, then take it out to thaw, easy way to get most of the sediment out, if you reduce it on a low heat and repeat you will probably get even more out
|
|
|

DMT-Nexus member
Posts: 190 Joined: 24-Jan-2011 Last visit: 21-Mar-2013 Location: My body for now
|
And I egg clarified it before I filtered it. I will give the freezer thing a go on the next batch. I think I will use fumeric also. The truth is not for all men, but only for those who seek it.
|
|
|

DMT-Nexus member
Posts: 121 Joined: 05-May-2008 Last visit: 24-Jan-2025 Location: 2nd star to the right
|
back to what fractal enchantment was suggesting..........what if one were to freebase a vinegar water solution using sodium carbonate and then saturate the solution with non-iodized salt ? freebase dmt being insoluble in water should precipitate out right ? or would the dmt react with the salt creating an hcl and redissolve in the water ? if so, what about saturating the water with sodium bicarb ? since water can only hold so much sodium the excess should push the freebase out...yes ?
extracting with vinegar is a nice easy way to get a clean and strong dmt solution, but how to get the salt out of that solution without using fancy solvents is still perplexing me.
|
|
|

DMT-Nexus member
Posts: 206 Joined: 12-Jul-2010 Last visit: 15-Oct-2024
|
Maybe with a highly water-soluble salt?
I think the manske works because the NaCl is more water-soluble than the harmalas, so it dissolves and "kicks out" the harmalas.
Or does the Na+ ion/ Cl- ion combine with the harmala ion?
Maybe you just need to find a salt more water-soluble than harmalas?
Maybe not?
|
|
|

DMT-Nexus member
Posts: 1999 Joined: 13-Jun-2011 Last visit: 24-Jun-2018
|
intosamadhi wrote:I think this post is just going to illustrate my lack of chem know how.....
rather than aiming to create dmt hcl, was hoping that the water would just become so saturated with sodium chloride that it would sort push out the dmt acetate......
but that would only happen if dmt acetate is less soluble than sodium chloride right?.....looking at a solubility table it seems that acetates in general are pretty water soluble......so not an effective method of precipitation........
while on this topic though (maybe not the proper place for it but...) ..... i can't find mentioned in the forum of anyone performing a straight vinegar & sodium carbonate extraction on mimosa......so soaking some powdered MHRB in pure 5% distilled vinegar and then adding carbonate, filtering and collecting the mud.......would all the spice end up in the mud or would some still remain in the water ? (the idea here, again, being not to create the purest product but just something a bit more concentrated and less nauseating than a tea)
SWIM has tried drinking the water (because he thought all the dmt would remain in the water and only the tannins and other crud would fall to the bottom) of such a reaction with mixed results.....if drunk a day or so after adding sodium bicarb (so not sodium carbonate)....it is still active, but such a mixture left in the fridge for several weeks makes the water inactive...........any thoughts? (again sorry if this is the wrong place....saw 'salting' and thought it was relevant) Why not just do a regular extraction, pull with D-Limo and salt with vinegar? Same outcome.... Lose Control, Free My Soul, Break Me Open, Make Me Whole."DMT kicked my balls off" - od3
|
|
|

Kalt und Heiß, Schwarz und Rot, Kürper und Geist, Liebe und Chaos
 
Posts: 4661 Joined: 02-Jun-2008 Last visit: 30-Apr-2022
|
Manske precipitation works because the hydrochloride salts of harmalas are not very water-soluble, especially in fridge temperatures and most importantly in the presence of an excess of chloride ions (as it happens in the presence of 10%+ sodium chloride). The latter is a result in the shift in dissolution equilibrium of harmala hydrochlorides. The same could happen with dmt, but the apparent high solubility of dmt-HCl won't make manske work in theory. And in practice, people have tried in the past and no, it doesn't work. Maye if one knew a dmt salt with poor solubility in water, then some good strategy to precipitate it could be devised. Need to calculate between salts and freebases? Click here! Need to calculate freebase or salt percentage at a given pH? Click here!
|